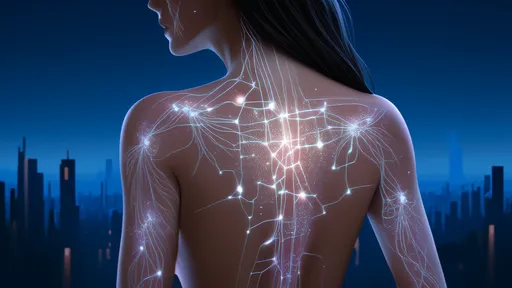
In the shadowy intersections of biology and neurology, a peculiar discovery has begun to rewrite our understanding of communication networks—not just among neurons, but between entirely different kingdoms of life. The term "neuro-mycelial web" has emerged to describe the eerie parallels between fungal mycelium and the human brain’s neural architecture. But recent studies suggest these parallels might be more than metaphorical. Researchers are uncovering evidence that fungi and brain cells might engage in a form of cross-kingdom signaling, a dialogue conducted through biochemical whispers we’re only beginning to decipher.
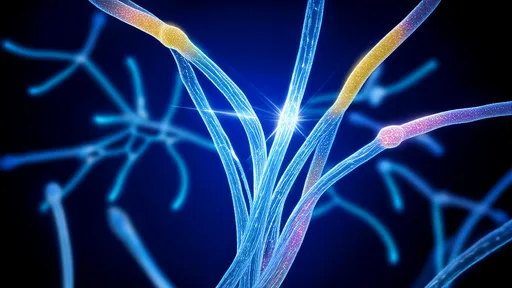
The mycelium—a vast, thread-like network of fungal hyphae—has long been dubbed nature’s internet, transmitting nutrients and information across ecosystems. Its structure bears an uncanny resemblance to the brain’s neural networks, with nodes and branches that pulse with electrical activity. Now, experiments reveal that certain fungi respond to neurotransmitters like serotonin and dopamine, chemicals once thought exclusive to animal nervous systems. Even more startling, fungal networks appear to alter their growth patterns in response to these signals, suggesting a rudimentary form of recognition or even memory.
What does this mean for neuroscience? For decades, the brain’s complexity was attributed to its cellular specialization. But if fungi—organisms without neurons—can exhibit brain-like behaviors, the very definition of cognition may need expansion. Some theorists propose that intelligence isn’t confined to neurons but is instead a property of any sufficiently interconnected system. This idea, dubbed "basal cognition," challenges the anthropocentric view of intelligence and raises provocative questions about how life processes information.
Laboratory experiments have added fuel to this fire. When exposed to neural stimuli, fungal hyphae have been observed forming dense clusters around electrodes, as if drawn to the source. Electrophysiological recordings detect spikes of activity resembling action potentials, though slower and less predictable than those in animal cells. Meanwhile, brain tissue cultures exposed to fungal compounds show altered synaptic plasticity—the foundation of learning and memory. The implications are as unsettling as they are profound: could there be a silent, ancient language shared between fungi and neurons?
The medical ramifications loom large. Fungi are already notorious for their ability to hijack host biology, as seen in infections like Candida auris. But what if some species exploit neurological signaling to manipulate behavior? Ophiocordyceps, the "zombie-ant fungus," is infamous for controlling insect hosts. Could analogous mechanisms exist in mammals? Conversely, might we harness fungal networks to repair neural damage? Early-stage research explores using mycelial scaffolds to guide axon regeneration in spinal cord injuries, leveraging their natural penchant for creating intricate pathways.
Beyond medicine, this research destabilizes the boundaries we impose on nature. The mycelial web beneath our feet and the neural web inside our skulls may operate on similar principles, hinting at universal rules of information processing. Philosophers and scientists alike are left grappling with a radical possibility: that intelligence, in its most elemental form, is a thread woven through all life, waiting to be untangled.
As labs worldwide race to map these cryptic connections, one thing becomes clear: the forest’s quiet fungal networks and the brain’s electric symphony may be verses in the same epic poem—one we’ve only just begun to read.
By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025
By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025
By /Jul 31, 2025

By /Jul 31, 2025

By /Jul 31, 2025
By /Jul 31, 2025
By /Jul 31, 2025
By /Jul 31, 2025
By /Jul 31, 2025

By /Jul 31, 2025